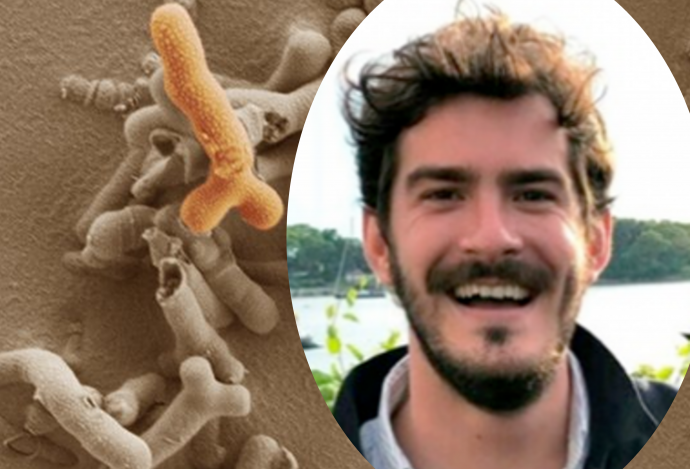
Researchers studying mice have found the first evidence of how a mother’s gut microbes can help in the development of the placenta, and the healthy growth of the baby
A new study published today by Hughes Hall Research Associate, Dr Jorge Lopez-Tello, et al, has found that a species of gut bacteria, known to have beneficial effects for health in mice and humans, changes the mother’s body during pregnancy and affects the structure of the placenta and nutrient transport – which impacts the growing baby.
The bacteria, Bifidobacterium breve, is widely used as a probiotic so this study could point to ways of combating pregnancy complications and ensuring a healthy start in life across the population.
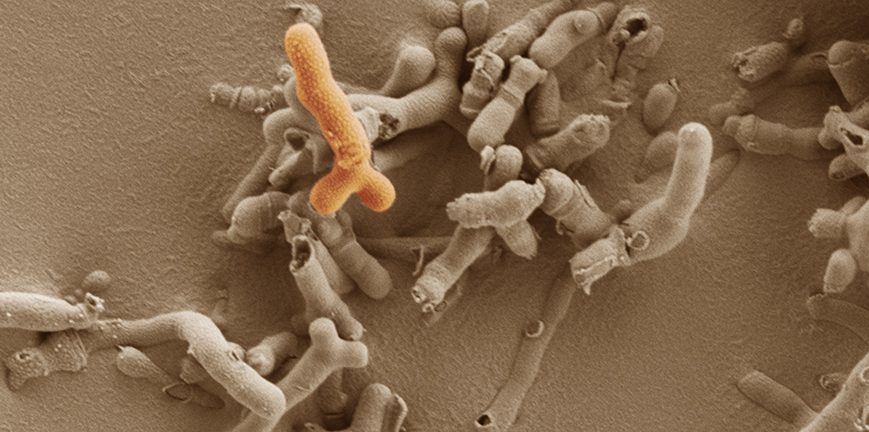
These Bifidobacterium bacteria rise in numbers in the microbiome during pregnancy in humans and mice, and alterations in its levels have been linked to pregnancy complications.
The research involved scientists from the University of Cambridge, the Quadram Institute, and the University of East Anglia and is published today in the journal Cellular and Molecular Life Sciences.
Microbes in our gut, collectively called the gut microbiome, are known to play a key role in maintaining health by combating infections, and influencing our immune system and metabolism. They achieve these beneficial effects by breaking down food in our diet and releasing active metabolites that influence cells and body processes.
Little is known about how these interactions influence fetal development and the baby’s health pre-birth. To address this, Professor Lindsay Hall from the Quadram Institute and University of East Anglia, and Dr Amanda Sferruzzi-Perri and Dr Jorge Lopez-Tello from the University of Cambridge analysed how supplementation with Bifidobacterium bacteria affected pregnancy in mice.
Hall has been studying Bifidobacterium and the microbiome in very early life, previously showing how providing specific probiotics can help premature babies. These bacteria rise in numbers in the microbiome during pregnancy in humans and mice, and alterations in its levels have been linked to pregnancy complications.
Sferruzzi-Perri said: “Pregnancy disorders affect around one in ten pregnant women. This is worrying, as pregnancy complications can lead to health problems for the mother and her baby even after the pregnancy.”
“This study, carried out in mice, identifies the maternal microbiome as a new player in the communication between mother, placenta and fetus. Finding out how this form of communication works and how to improve it may help many women who develop pregnancy complications, as well as helping their developing child.”

Dr Jorge Lopez-Tello, Hughes Hall Research Associate, and Dr Amanda Sferruzzi-Perri (above), both of University of Cambridge, carried out the study with Professor Lindsay Hall from the Quadram Institute and University of East Anglia.
‘Germ-free’ mice – lacking any microbes – can be bred to allow comparisons with other mice that have a ‘normal’ microbiome. This can provide valuable insights into the role of the microbiome in health – such studies can’t be carried out in humans.
In this study, the researchers also looked at the effect of feeding germ-free mice the probiotic Bifidobacterium breve.
In the germ-free mice, the fetus did not receive adequate sugar and failed to grow and develop properly. Excitingly, providing Bifidobacterium breve to germ-free mice improved fetal outcomes by restoring fetal metabolism, growth and development to the normal levels.
Lacking the maternal microbiome also hampered the growth of the placenta in a way that would affect fetal growth, and more detailed analysis identified a number of key cell growth and metabolic factors that appear to be regulated by the microbiome and Bifidobacterium breve.
“The placenta has been a neglected organ, despite it being vital for the growth and survival of the fetus. A better understanding of how the placenta grows and functions will ultimately result in healthier pregnancies for mothers and babies,” said Lopez-Tello.
The researchers also found that the microbiome affected key nutrient transporters, including those for sugars within the placenta that would also influence the growth of the fetus.
“Our findings reveal that the maternal microbiome promotes development of the placenta and growth of the fetus,” said Hall.
“We think that this is linked to the altered profile of metabolites and nutrients, which affects nutrient transport from mother to baby across the placenta. Excitingly it appears that adding in a probiotic Bifidobacterium during pregnancy may help to boost how the placenta functions, which has positive effects on the baby’s growth in the womb.”
These findings are strong indicators of a link between the microbiome of the mother and the development of the baby, but in this first study of its kind there are limitations.
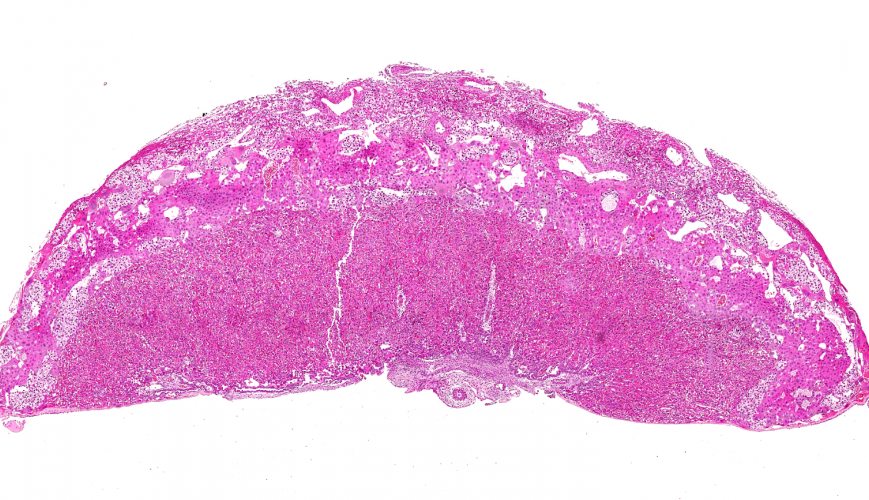
The study was carried out in mice and the image above shows a mouse placenta. “The placenta has been a neglected organ, despite it being vital for the growth and survival of the fetus” said Dr Lopez-Tello.
 This study focused on one single bacterial species, and whilst this showed that Bifidobacterium breve had positive effects on germ-free mice during pregnancy, this is not a natural situation. Future studies are needed to confirm these effects in a more natural and complex microbiome.
This study focused on one single bacterial species, and whilst this showed that Bifidobacterium breve had positive effects on germ-free mice during pregnancy, this is not a natural situation. Future studies are needed to confirm these effects in a more natural and complex microbiome.
The study was carried out in mice and cannot automatically be translated into treatments for humans. The knowledge provided in this proof-of-concept animal study is critical for guiding future studies in humans – to uncover whether the human maternal microbiome has similar effects. If that is the case, it could provide a relatively simple and low-cost way to help improve pregnancy outcomes with positive benefit for the life-long health of the mother and her child.
The research was funded by Wellcome and the Biotechnology and Biological Sciences Research Council.
Reference
- For the original article Maternal gut microbiota Bifidobacterium promotes placental morphogenesis, nutrient transport and fetal growth in mice, see https://link.springer.com/article/10.1007/s00018-022-04379-y
- Lopez-Tello, J. et al: ‘Maternal gut microbiota Bifidobacterium promotes placental morphogenesis, nutrient transport and fetal growth in mice.’ Cellular and Molecular Life Sciences, June 2022.
- Adapted from a press release by the Quadram Institute.
- About Jorge Lopez-Tello: https://www.hughes.cam.ac.uk/about/our-people/seniors-members/jorge-lopez-tello/.
28.6.22